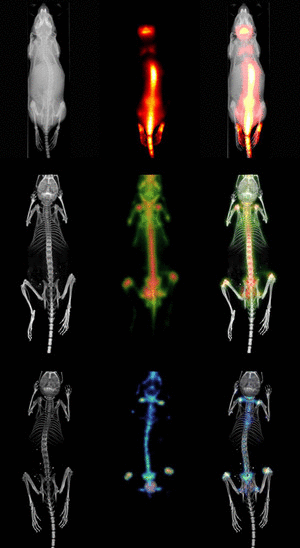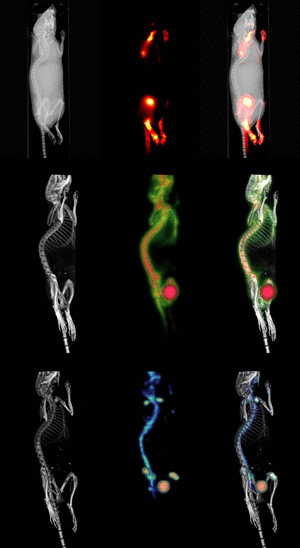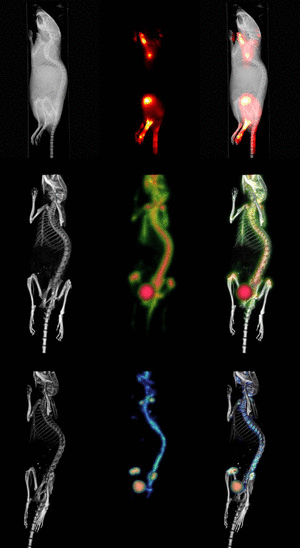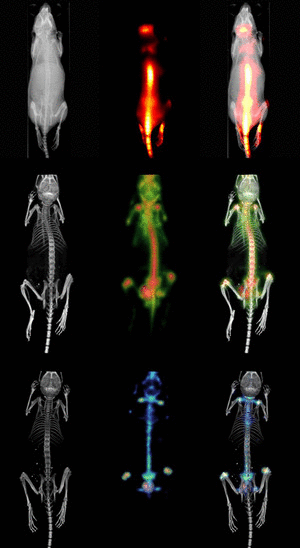
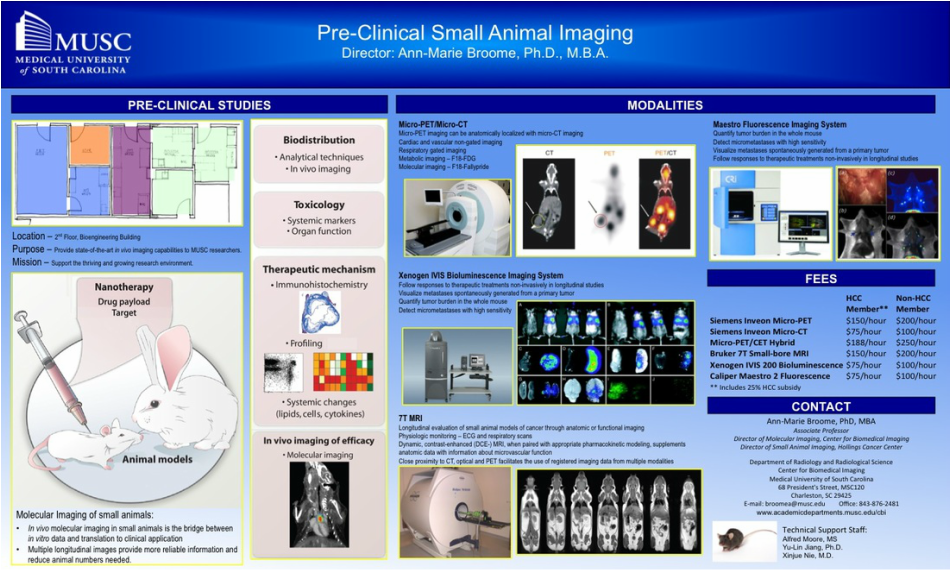
Molecular Imaging
|
The field of molecular imaging emerged in the early 1990’s as scientists from multiple disciplines, including cell biology, biomedical engineering, chemistry, mathematics, medicine, pharmacology and genetics began working toward the development of imaging instruments, imaging probes, assays, and quantification techniques to elucidate molecular mechanisms in biology and medicine. Molecular imaging aims to non-invasively visualize, characterize and quantify normal and pathologic processes within the living organism at the cellular and subcellular level. E.A. Zerhouni, MD, former director of the National Institutes of Health, has described molecular imaging as having “…the potential to define itself as a core interdisciplinary science for extracting spatially and temporally resolved biological information at all physical scales from Angstroms to microns to centimeters in intact biological systems.” (Eugene P. Pendergrass New Horizons Lecture, Radiological Society of North America meeting, 2007)(1). Even in its early stages of development, molecular imaging is revolutionizing our ability to see and monitor specific proteins and genes, and characterize molecular pathways within the living organism.
In contrast to traditional biomedical imaging by microscopy, in which excised tissues are typically examined to characterize histological changes and thus identify an underlying disease process, molecular imaging targets distinct molecular pathways in vivo, providing visual and quantitative information for diverse research applications. For example, investigators have applied molecular imaging to: 1) noninvasively characterize the stages and progression of a disease process and establish signature biomarkers; 2) assess the efficacy of standard or experimental treatment modalities in small-animal models of human disease; 3) characterize the trafficking of stem cells and immune cells; 4) analyze the biodistribution of drugs and the dynamics of drug/receptor interactions; 5) investigate the cellular and subcellular basis of brain disorders; 6) assess metabolic changes, particularly in the brain, heart and tumors; and 7) detect tissue hypoxia. Because biological systems are, at one level, highly complex and dynamically changing assemblies of molecular structures and interactive pathways, molecular imaging provides the tools to investigate in real time the distribution and activity of targeted biomolecules as they undergo changes in space and time. Molecular imaging offers scientists in many different specialties significant advantages over traditional research paradigms. First, molecular imaging procedures can be conducted in the living organism. Whereas studies of tumor responsiveness to a new therapeutic agent, for example, would have traditionally involved a large cohort of animals from which subsets would be analyzed histologically at multiple time points, molecular imaging allows characterization of tumor development and response to a therapeutic, and even response to discontinuation of the therapeutic, within the same small set of animals imaged longitudinally at multiple time points. This example illustrates two associated advantages of molecular imaging: 1) a study can be conducted with significantly fewer animals (thereby minimizing animal usage and reducing animal costs) than would otherwise be necessary; and 2) the statistical power is increased because each animal serves as its own control. Other advantages include the ability of molecular imaging procedures to interrogate the whole body, in addition to focusing on specific regions, and to visualize the molecular target of interest in 3-dimensional space. Finally, molecular imaging is becoming a key bridging technology to translate experimental preclinical findings into the clinical environment. |